Container Closure Integrity Testing of Blister Packs
Container Closure Integrity Testing (CCIT) plays a vital role in maintaining the integrity of blister packs in pharmaceutical packaging. In this article, we look into the innovative CCIT methods that have been developed to overcome the limitations of traditional dye ingress testing. We explore how these advanced techniques provide a more robust and accurate solution for ensuring the quality control of pharmaceutical primary packaging.
What is CCIT for blister packs?
CCIT, or Container Closure Integrity Testing, is a crucial process in pharmaceutical packaging designed to ensure product quality during shelf life. It is a quality control process used to detect leaks, defects, or weak seals in the packaging that could potentially compromise the quality and safety of the enclosed pharmaceutical products. When it comes to blister packs, CCIT involves specialised testing techniques to identify defects in the blister packs to prevent contamination and moisture ingress. These defects may arise from issues like faulty seals, cavity defects, material issues or blister packaging machine settings.
What are the different leak test methods for blister packs?
Pharmaceutical manufacturers utilise a variety of techniques to ensure the integrity of blister packs, each tailored to meet specific needs and quality assurance requirements.
- Dye Ingress Testing
The most widely used method is dye ingress testing. This method involves immersing a selection of blister packs in a solution of blue dye and water, and then subjecting the packs to a vacuum of typically 200-600mbar for several minutes. After the vacuum is released, any defective pockets in the blister packs will absorb the blue dye, allowing them to be easily identified. The packs are opened and inspected for any signs of dye ingress. Dye ingress testing is cheap and easy to perform, however the test is subjective, time-consuming and destructive.
- Tracer gas (vacuum mode): F2391−22
Leak detectors based upon mass spectrometers detect and quantitively measure the leakage rate of tracer gases such as hydrogen or helium. Test samples are flooded with tracer gas and placed inside an vacuum chamber. When the chamber is evacuated, any leaking gas is drawn through the analyser. This is a highly sensitive testing method, but the requirement for gas makes it quite expensive.
-
Force Decay
Another method is force decay. This method can be used by measuring the decay in force applied to the surfaces of the packs over time. By pressurising the packs, any drop in pressure indicates a leak. The force decay method is a non-destructive option that provides deterministic results. - Vacuum Deflection by laser measurement: F3169-16 (2024)
The most advanced technology is vacuum deflection by laser measurement that assesses blister packs by scanning their surfaces with a laser before and after applying vacuum. This technology accurately measures the deflection or height difference in the foil of each cavity, under vacuum. When there are no holes present, the foil reacts by moving and causing deflection. However, if there's a large hole, there is minimal deflection as the pressure equalises. Small holes, on the other hand, display initial expansion under vacuum. To identify these small holes, a third scan is needed. As the vacuum is reduced, any increased collapse signifies the presence of a small hole, where air escapes gradually, equalising the pressure inside the pocket with the applied vacuum.
What regulatory compliance should be considered?
Good leak detection procedures are a critical element of GMP (Good Manufacturing Practice) and provide the manufacturer with confidence over the integrity of their packs, as well as ensuring product stability and overall shelf life.
Different ASTM standard test methods can be referenced including ASTM F3169-16 (2024) and ASTM F2391-22.
ASTM F3169-16 (2024) is the standard test method for leak detection in blister packaging by vacuum deflection method by laser measurement. This method is used for non-porous blister packs sealed with flexible lidding material. These typically consist of thermoformed polymer or cold formed aluminium trays that contain tablets or capsules in individual blister pockets. The formed trays can be sealed with a polymer, foil or paper lidding material.
ASTM F2391-22 is the standard test method for measuring package and seal integrity using helium as the tracer gas. This method can be used for leak testing cold-formed aluminium blister packs.
Why should companies consider non-destructive leak testing?
Detecting leaks in blister packaging has traditionally involved destructive test methods like dye ingress, but demand for non-destructive technologies, which do not result in wasted products, is slowly increasing. Non-destructive leak test methods can reduce unnecessary and costly waste as packs that have passed the test can be returned to the line or product can be recovered to be repackaged. On the other hand, packs that have .jpg?width=294&height=184&name=Types%20of%20Blister%20Packaging%20(2).jpg) been tested using destructive methods like dye ingress, will need to be destroyed whether they pass the test or fail. Depending on the value of the drugs inside the packs and the number of packs tested per hour, the cost savings can be substantial. Manufacturers of expensive drugs such as oncology drugs could potentially save tens of thousands of pounds, euros or dollars every week during batch testing.
been tested using destructive methods like dye ingress, will need to be destroyed whether they pass the test or fail. Depending on the value of the drugs inside the packs and the number of packs tested per hour, the cost savings can be substantial. Manufacturers of expensive drugs such as oncology drugs could potentially save tens of thousands of pounds, euros or dollars every week during batch testing.
Furthermore, with an increasing focus on sustainability, pharmaceutical manufacturers are actively seeking ways to minimise their environmental impact. Switching to non-destructive methods could be a much greener choice as it significantly reduces product waste and eliminates the need for incineration. Unlike traditional methods, non-destructive testing does not require a daily supply of water, avoiding any potential contamination risk and eliminating the associated costs of water treatment and disposal.
What is the standard acceptable leak size for CCIT of blister packs?
The acceptable leak size for Container Closure Integrity Testing of blister packs is not standardised and can vary depending on factors including the type of product, packaging material, and the specific requirements of the pharmaceutical manufacturer. There is no universal measurement for leak size in this context.
Regulatory bodies including the U.S. Food and Drug Administration (FDA) and the European Medicine Agency (EMA) do not specify a fixed leak size. Instead, they emphasise the importance of pharmaceutical manufacturers demonstrating the integrity of their packaging by utilising leak test methods that are sensitive enough to detect defects that could compromise product quality and safety.
Manufacturers typically set their standards for leak size based on the unique characteristics of their products and packaging materials. The necessary level of test sensitivity is determined through rigorous testing and validation procedures, guaranteeing that the chosen leak test method effectively identifies any leaks that could impact the integrity of the product.
What are the limitations of dye ingress testing?
Dye ingress testing of blister packs has several limitations, particularly when it comes to its sensitivity in detecting small leaks. Below are some of the main limitations to keep in mind:
- Limited sensitivity
The sensitivity of dye ingress testing is often limited, making it challenging to detect micron holes or very small defects in the packaging. This limitation can potentially result in false negatives, compromising the accuracy of the test. - Subjective interpretation
The results from dye ingress testing are subjective and can be prone to error. The interpretation of the test depends on the observation of the operator, which could lead to inconsistencies in how the results are interpreted. - Destructive method
The dye ingress test method is a destructive method. All packs are submerged in blue dye, requiring all packs, including packs that have passed the test, to be destroyed.
- Mess & potential contamination
Dye ingress testing can create a messy environment, leading to potential contamination concerns, especially in clean production areas where maintaining sterility and cleanliness is paramount.
- Environmental implications
The use of coloured dyes can raise concerns regarding the impact on the environment due to issues related to waste disposal, making it a less eco-friendly option compared to non-destructive leak test methods. Moreover, incineration of waste product could be environmentally damaging.
Why choose deterministic, more expensive CCIT systems over dye ingress?
Although dye ingress offers a much cheaper leak test solution, the critical importance of package integrity and reliability in the pharmaceutical industry makes non-destructive and deterministic methods the preferred choice. These methods offer higher sensitivity and accuracy which is crucial to meeting stringent regulatory requirements and maintain the highest quality control.
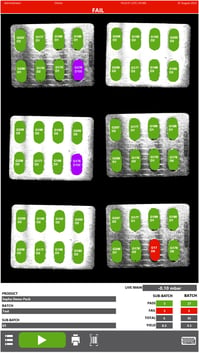 By investing in advanced deterministic leak test methods, manufacturers can uphold the highest standards of quality control, meet regulatory requirements, and ensure the safety of pharmaceutical products. It can also mitigate the risks associated with recalls, ensuring consumer safety and enhancing the overall reputation of the manufacturer. In contrast, the limitations of blue dye testing, including its non-deterministic lack of sensitivity and precision, make it an inadequate choice when it comes to safeguarding the safety and efficacy of medications.
By investing in advanced deterministic leak test methods, manufacturers can uphold the highest standards of quality control, meet regulatory requirements, and ensure the safety of pharmaceutical products. It can also mitigate the risks associated with recalls, ensuring consumer safety and enhancing the overall reputation of the manufacturer. In contrast, the limitations of blue dye testing, including its non-deterministic lack of sensitivity and precision, make it an inadequate choice when it comes to safeguarding the safety and efficacy of medications.
By embracing non-destructive leak test solutions that minimise waste and environmental impact, manufacturers have the opportunity to prioritise both the integrity of their packaging and their commitment to ecological responsibility. This not only demonstrates their dedication to high-quality production, but also reduces their overall environmental footprint, showcasing their commitment to sustainability.
What are the key benefits of the Sepha VisionScan 3D?
The VisionScan 3D uses innovative 3D Leak Detection technology to test the integrity of pharmaceutical blister packs. The tool-less system is a non-destructive and deterministic method that can test all foil types including matt and glossy finish, and busy text patterns. Key features and benefits include:
- Innovative 3D technology
Incorporates innovative 3D technology that can detect defects in individual blister pockets, channel leaks and weak seals as low as 5µm (pack and material dependent).
- ASTM F3169-16 (2024)
Utilises the ASTM test standard F3169-16 (2024) ‘Vacuum deflection by laser measurement’.
- Large test area
Can identify gross and micron holes in individual blister pockets, in multiple packs per test cycle. Large test area (297x210mm) provides high throughput.
- Tool-less
Tool-less device making it ideal for production lines running multiple products.
- Pack formats
Can test blister packs that contain tablets or capsules in multiple material/design formats.
- Validation
Streamlined validation process for different foil types or language versions.
- 21 CFR Part 11
Can form part of a 21 CFR Part compliant system.
- MES Integration
The VisionScan 3D can be easily linked to a MES via its MES connector. This enables manufacturers to control production, monitor and analyse processes, and document data and activities to increase efficiencies.
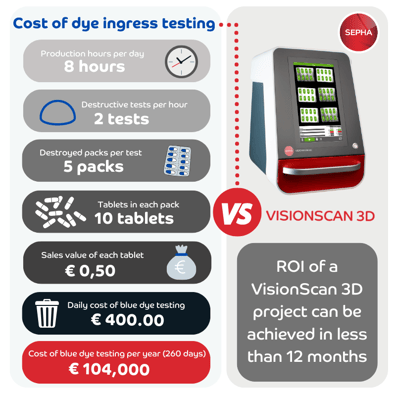
Cost of dye ingress testing vs purchase of VisionScan 3D?
Typically, the return on investment (ROI) for a VisionScan 3D project can be achieved in less than a year. The parameters used in the infographic below are conservative, assuming only 2 batch tests per hour with 5 blister packs each, based on a 5-day work week. However, the ROI can be achieved much quicker when testing more blister packs at regular intervals or when dealing with higher-value drugs. In fact, we have had the pleasure of working with manufacturers who achieved ROI in less than 3 months. Read more on 'The cost savings of VisionScan 3D vs dye ingress testing'.
Interested to learn more?
More information can be found on the VisionScan 3D product page or via the on-demand webinar ‘3D Leak Detection of Blister Packs’.
If you would like to discuss your blister leak test requirements in more detail, please get in touch on +44(0)2890 48 48 48, +1 616-888-9081(USA) or via the button below.
Other interesting articles:
-
Is blue dye ingress testing letting you down in your green efforts?
- Is USP 1207 relevant to blister leak test procedures?
- Pharmaceutical Packaging - the silent bodyguard