Vacuum Decay Testing of Parenterals
What is vacuum decay testing?
Vacuum Decay Testing is a non-destructive and deterministic leak test method to identify leaks in pharmaceutical containers. To conduct a test, a container is placed in a tightly fitted chamber which is evacuated to a predetermined level of vacuum. After reaching the pre-set vacuum, a sensor measures the vacuum level over a predetermined time.
 If the integrity of the container under test has been compromised (it has a leak), there will be a rise in pressure measured inside the chamber. This will result in a) a gross leak, if the vacuum doesn’t reach the pre-set vacuum level; b) a medium leak, if the vacuum level drops below a pre-set vacuum level during the test or c) a decay or micron leak, if the vacuum level exceeds the decay limit during the test time.
If the integrity of the container under test has been compromised (it has a leak), there will be a rise in pressure measured inside the chamber. This will result in a) a gross leak, if the vacuum doesn’t reach the pre-set vacuum level; b) a medium leak, if the vacuum level drops below a pre-set vacuum level during the test or c) a decay or micron leak, if the vacuum level exceeds the decay limit during the test time.
Four types of containers/packages can be tested using the vacuum decay test method in accordance with the ASTM F2338-09 standard :
- Rigid, non-porous containers like glass or plastic vials, ampoules, pre-filled syringes or ophthalmic bottles.
- Flexible, non-porous packages including bags and pouches
- Rigid and semi-rigid non-lidded trays*
- Trays or cups sealed with porous barrier lidding material*
* Must be masked or blocked during testing to create a completely sealed package
Choosing a robust CCIT method
As a manufacturer of pharmaceutical containers and medical devices you are required to demonstrate the integrity of your container and closure systems (CC). The CC systems need to protect sterile products from potential contamination to ensure product safety throughout its shelf life.
 As it is the type of drug that will define the maximum allowable leak size and required test sensitivity, it is key to choose the right Container Closure Integrity Test (CCIT) method during the drug development stage. The Sepha Multi-Q is a non-destructive and deterministic CCIT device that has been specially developed to test the integrity of parenterals (including vials, ampoules, bottles and pre-filled syringes), flexible packaging and medical device trays. The multi-functional machine operates in combination with different test methods including vacuum decay, but also pressure decay.
As it is the type of drug that will define the maximum allowable leak size and required test sensitivity, it is key to choose the right Container Closure Integrity Test (CCIT) method during the drug development stage. The Sepha Multi-Q is a non-destructive and deterministic CCIT device that has been specially developed to test the integrity of parenterals (including vials, ampoules, bottles and pre-filled syringes), flexible packaging and medical device trays. The multi-functional machine operates in combination with different test methods including vacuum decay, but also pressure decay.
Depending on container type, container content and the leak rate sensitivity that needs to be achieved, our technical team will develop a method for vacuum decay testing your containers. Containers that cannot be tested using a vacuum decay test, including toxic product or oil-based liquids, can be set up for pressure decay testing.
Parenteral applications that have a lower Maximum Allowable Leakage Limit (MALL) often require a higher level of sensitivity. The Multi-Q HD has been developed to reach this sensitivity enabling ultra-sensitive detection of micron defects as low as 1µm.
Multi-Q Vacuum Decay Leak Test Process
Once vacuum decay has been established as the appropriate CCIT method for your container, tooling needs to be designed and a method needs to be developed in line with the desired sensitivity requirements. A tooling library is available for standardised container sizes. For non-standard or complex containers, custom tooling may be required to ensure the container fits the chamber with a tight tolerance. This is important as it reduces the head space within the vacuum chamber, resulting in a more accurate and robust test method.
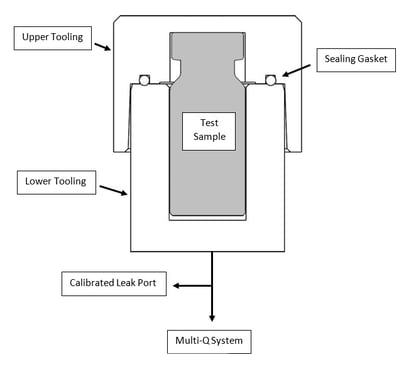 Fig. 1 Vacuum Decay Leak Test Tool
Fig. 1 Vacuum Decay Leak Test Tool
To validate the method, calibrated leak standards are used to verify the minimum detectable leak rate. Once the test method is set up, a quick and easy 4-step process follows.
- Placing the sample
The sample container is placed into the bespoke tooling. The upper tool is placed on top of the lower tool, enclosing the sample. - Applying Vacuum
Once the sample is enclosed in the chamber, a vacuum is applied. - Vacuum Measurement
The vacuum level inside the chamber is monitored for a predetermined time using absolute and/or differential pressure transducers. - Pass/Fail Result
An increase in pressure that exceeds the set threshold will result in a ‘fail’. If the pressure level does not rise above the threshold then the sample under test will result in a ‘pass’.
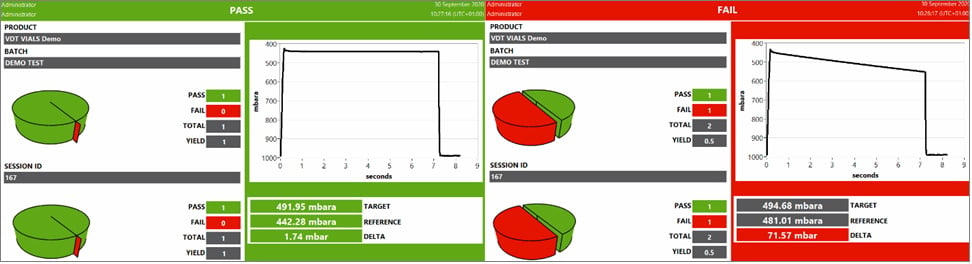
Fig. 2 Vacuum Decay Deterministic Pass / Fail results
ASTM F2338-09 and USP1207
The Multi-Q utilizes the FDA approved ASTM F2338-09 vacuum decay leak test method to perform the test. This method is also referenced in the revised USP 1207 guidance in which deterministic methods are preferred over probabilistic procedures such as the bubble test or blue bath method. The revised chapter will be applied to vials, ampoules, syringes, and bags.
Multi-Q Features & Benefits
- Vacuum Decay leak test method to identify leaks and channel leaks as low as 5µm (dependent on container type and content)
- The Sepha Multi-Q HD can test down to 1µm (dependent on container type and content) and has been developed for parenterals that require extra sensitivity
- Non-destructive and deterministic test method
- Ideal for testing bottles, ampoules, vials, BFS strips & bottles, medical devices, pre-filled syringes, pouches and trays
- Can test containers with a water-based liquid
- Rapid test time as low as 10 seconds
- Reliable and repeatable results
- Utilizes the ASTM F2338-09 test method to perform the leak test
- In line with USP 1207 guidance
- 21 CFR Part 11 compliant
Interested in Vacuum Decay Testing?
Are you interested in Vacuum Decay Testing but unsure whether the Sepha Multi-Q test method is right for your container? Contact us for expert advice & feasibility studies to assess product and container compatibility.
Get in touch on+44(0)28 9048 4848 or use the form on our Contact page, our sales team and engineers will be happy to discuss your requirements in more detail. Alternatively, download the Multi-Q Brochure or Multi-Q HD brochure for more detailed information and technical specifications.
Watch Multi-Q Vacuum Decay Video
Other interesting articles:
- 'USP 1207: Opening the CCIT Toolbox'
- 'Container Closure Integrity Testing (CCIT): Safety in numbers'
- 'Quality Control Test for Parenterals: a guide to choosing a compliant leak tester'


